Abstract
Interstitial deletions on chromosome 5q [del(5q)] are the most common cytogenetic alteration in patients with myelodysplastic syndrome (MDS), affecting up to 25% of patients. The size of the deletion and the breakpoints vary among patients and include two commonly deleted regions (CDR). The distal CDR is located between 5q32-5q33 and the proximal CDR is located on 5q31. Several genes in the CDRs have been implicated in del(5q)-associated disease pathogenesis through haploinsufficiency, including RPS14, SPARC, miR-145 and miR-146 in the distal CDR, and EGR1, CTNNA1, and HSPA9 in the proximal CDR. Most patients have deletions that span both CDRs, therefore, we asked whether simultaneous deletion of genes on both CDRs cooperate to contribute to altered hematopoiesis.
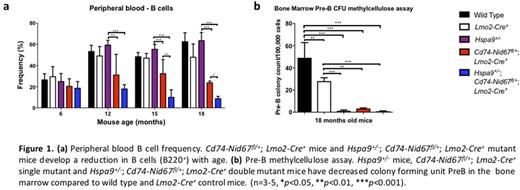
In this study, we crossed Hspa9+/- mice (a gene on the proximal CDR) with Cd74-Nid67fl/+ mice (loxP sites flanking 8 distal CDR genes , Nid67, Dctn4, Rbm22, Myoz3, Synpo, Ndst1, Rps14 and Cd74) . Cd74-Nid67fl/+ mice also express the Lmo2-Cre transgene that is expressed during embryonic hematopoiesis. We comprehensively characterized the hematopoiesis in (1) wild type control mice, (2) Hspa9+/- mice, (3) Lmo2-Cre+ mice, (4) Cd74-Nid67fl/+ ; Lmo2-Cre+ mice, and (5) double mutant Hspa9+/- ; Cd74-Nid67fl/+ ; Lmo2-Cre+ mice. We observed the previously reported reduction in bone marrow colony forming unit-preB (CFU-preB) colony formation in Hspa9+/- single-mutant mice. We also observed the reported anemia with decreased RBC maturation (n=4-8, P<0.05) and increased apoptosis in bulk bone marrow cells (n=4-8, P<0.05) in Cd74-Nid67fl/+ ; Lmo2-Cre+ (Cd74-Nid67+/-) mice. At 18 months of age, Cd74-Nid67+/- mice also developed reduction in B cell frequency in the bone marrow (BM) (≥2.1-fold, n=3-5, P<0.01), spleen (≥1.4 -fold, n=3-5, P<0.05) and peripheral blood (≥2.0-fold, n=3-5, P<0.05) (Figure 1a) that was associated with reduction in CFU-preB methylcellulose colonies (≥8.4-fold, n=3-5, P<0.001) compared to wild type and Lmo2-Cre+ control mice (Figure 1b). Hspa9+/- ; Cd74-Nid67fl/+ ; Lmo2-Cre+ double mutant mice showed a reduction of CFU-PreB methylcellulose colonies compared to wild type and Lmo2-Cre+ control mice (≥42.4-fold, n=3-5, P<0.001) (Figure 1b). Hspa9+/- ; Cd74-Nid67fl/+ ; Lmo2-Cre+ mice also developed a reduction in common lymphoid progenitors (≥1.6-fold, n=3-5, P<0.05) and Hardy B cell fractions A-E (≥3.4-fold, n=3-5, P<0.05) in the BM, with at least a 2-fold reduction in mature B cell frequencies in the BM, spleen and peripheral blood (n=3-5, P<0.05) compared to wild type, Lmo2-Cre+ and Hspa9+/- control mice (Figure 1a). These data suggest that haploinsufficiency of Hspa9 cooperates with haploinsufficiency of one or more genes deleted in Cd74-Nid67+/- mice to reduce B cells. We next asked which of the 8 genes on the Cd74-Nid67 deleted interval contributed to the B cell defect.
CD74 is a transmembrane protein expressed on antigen presenting cells and mediates major histocompatibility (MHC) class II assembly and trafficking. It has been suggested to regulate mature B cell survival through macrophage migration inhibitory factor (MIF) signaling. To better understand the role of CD74 in hematopoiesis, we characterized B cells in Cd74 knockout mice. We observed that Cd74 homozygous and heterozygous knockoutmice (2-3 months old) have decreased CFU-PreB colonies in BM compared to wild type control mice (4.4-fold, n=4, P<0.01). The Cd74-/- mice, but not Cd74+/- mice, also have decreased peripheral blood B cells (1.5-fold, n=4, P<0.01) at 3 months. This result indicates that loss of Cd74 likely contributes to the B cell phenotypes observed in the Cd74-Nid67+/- mice. We predict that heterozygous loss of Cd74 and Hspa9 will cooperate to induce similar B cell phenotypes observed in Hspa9+/-; Cd74-Nid67+/- double mutant mice. These studies are ongoing by intercrossing Cd74+/- and Hspa9+/- mice, as well as mechanistic studies to understand how Hspa9 and Cd74 haploinsufficiency regulate B cell development. Collectively, these studies may provide insight into B cell alterations that occur in MDS patients with del(5q).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal